If you work for a facility designated as a Certified Comprehensive Stroke Center, are you ready for the Comprehensive Stroke measure changes going into effect on January 1, 2018?
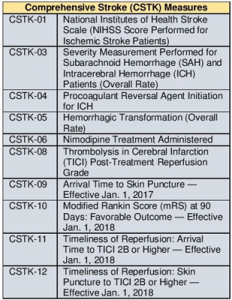
Below is an outline of the measures being suspended and new measures being added for data collection in 2018. Please familiarize yourself with these changes.
- Suspending CSTK-02: Modified Rankin Score (mRS) at 90 Days. Originally intended as an outcome measure, CSTK-02 has focused on the process of obtaining score data 90 days after the patient’s discharge from the hospital. Comprehensive Stroke Centers now have processes in place to collect 90-day mRS data with aggregate performance nearing 90%.
- With that in mind, CSTK-10: Modified Rankin Score (mRS) at 90 days: Favorable Outcome, will be added to the CSTK measures, replacing CSTK-02. This outcome measure captures the percentage of ischemic stroke patients treated with a reperfusion therapy (IV or IA thrombolytic [tPA] therapy or mechanical reperfusion [MER] therapy) and have a good outcome (mRS 0, 1, or 2).
The following new measures with be collected for CSTK cases, effective January 1, 2018 as well:
- CSTK-11: Timeliness of Reperfusion: Ischemic stroke patients who achieve TICI 2B or higher for the primary vessel occlusion within 120 minutes of hospital arrival.
- CSTK-12: Timeliness of Reperfusion: Ischemic stroke patients who achieve TICI 2B or higher for the primary vessel occlusion less than (<) or equal to 60 minutes from the time of skin puncture.